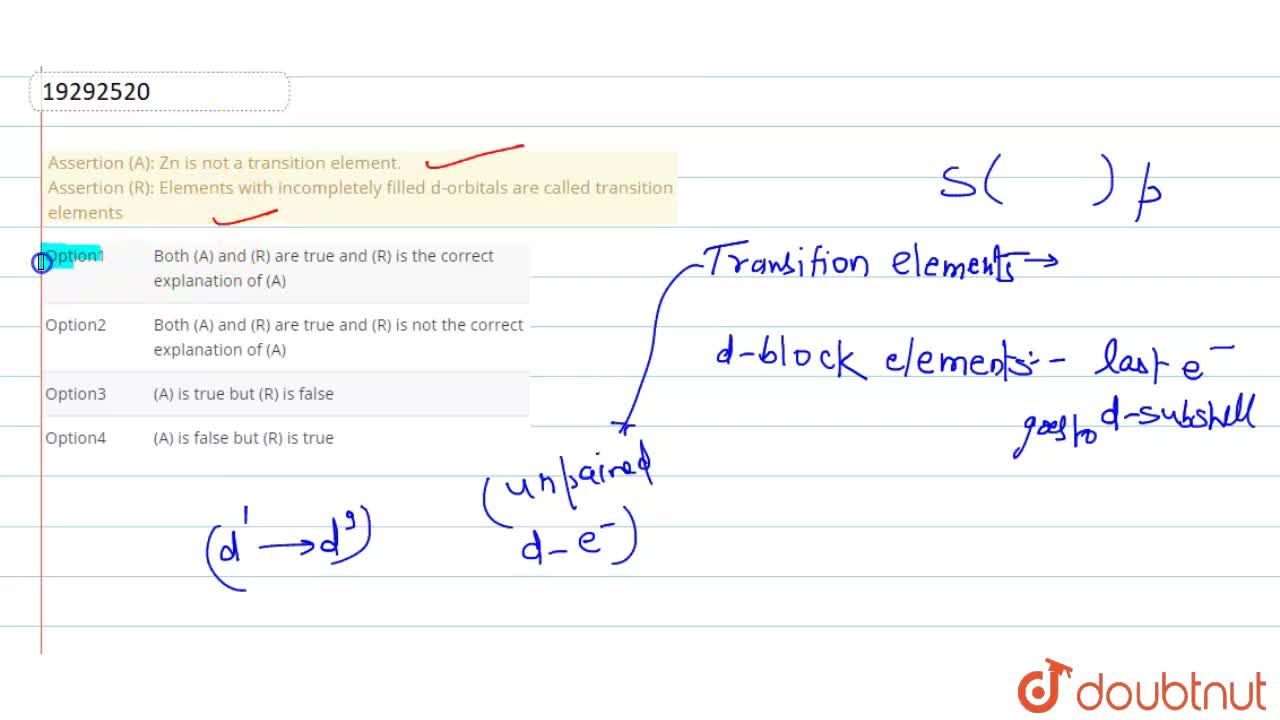
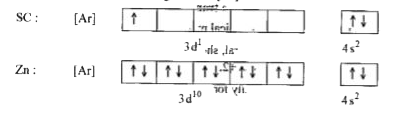
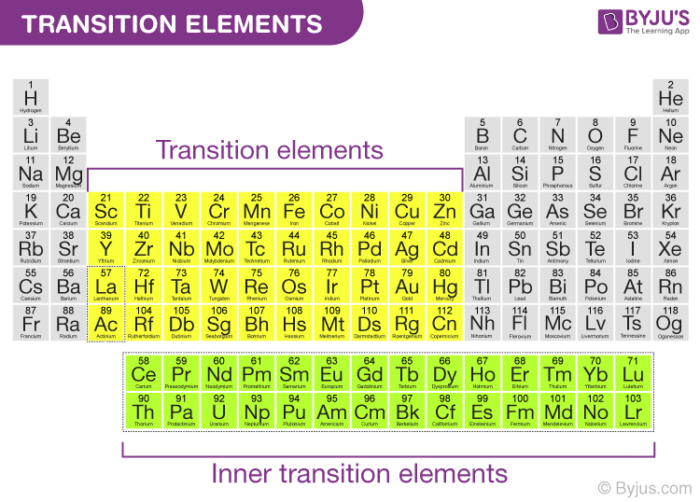
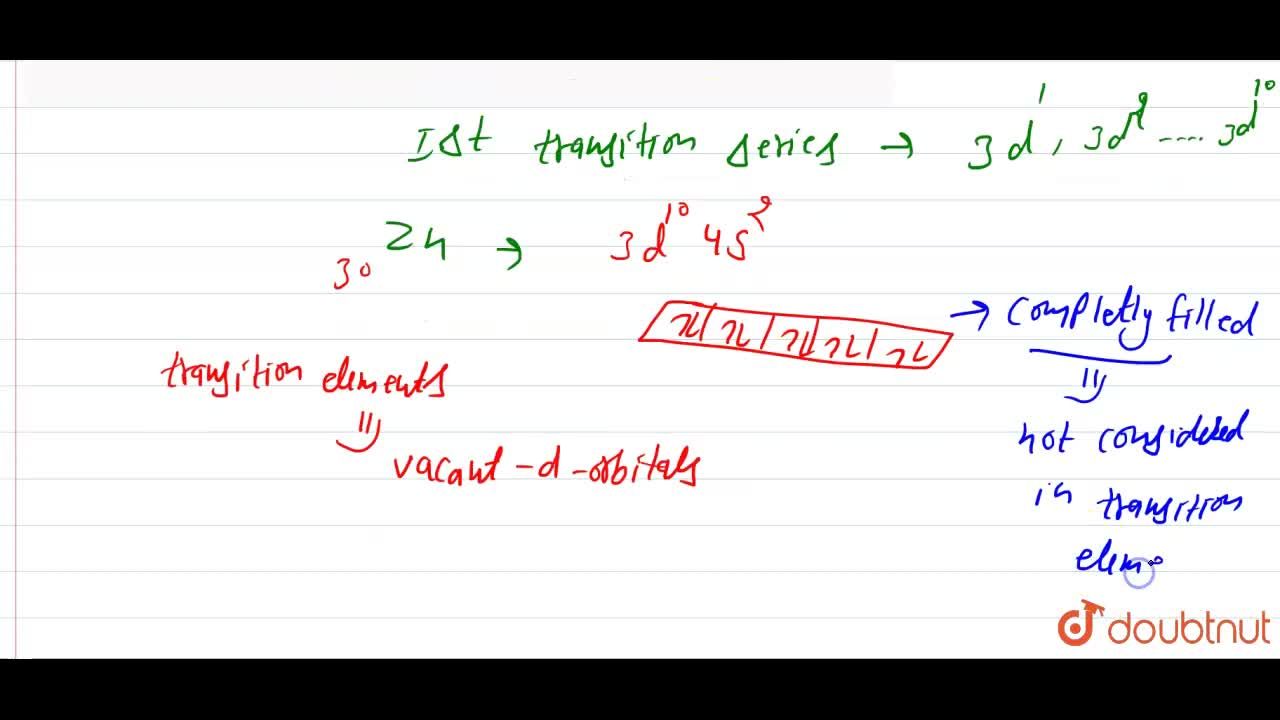
Explain the following observation: Zinc is not regarded as a transition metal - Chemistry - d- and f-Block Elements - 10669243 | Meritnation.com

Chapter 15: Transition Metals 15.1 General Properties of Transition Metals 15.2 Complex Formation and the Shape of Complex Ions 15.3 Coloured Ions ppt download
Why are scandium and zinc not regarded as transition metals despite their been in the transition series? - Quora

Class 12- D block element) Q.Why zinc is not considered as d block element or transition element? - YouTube

why are Zn,Cd & Hg not considered as transition elements | part 1 | Unit-8 |cbse|class 12 chemistry - YouTube

Why do the transition elements have higher enthalpies of atomization? In 3d series ( Sc to Zn ), which element has the lowest enthalpy of atomization and why?