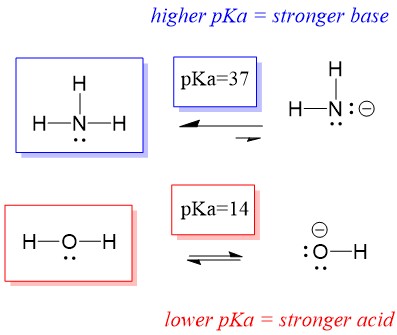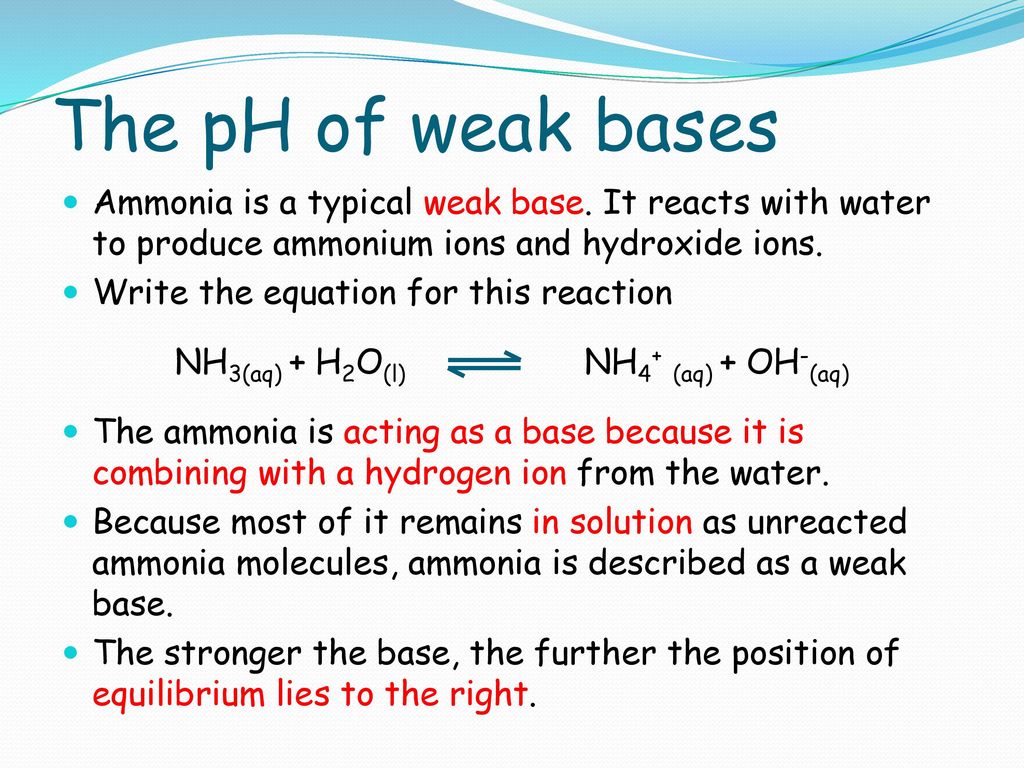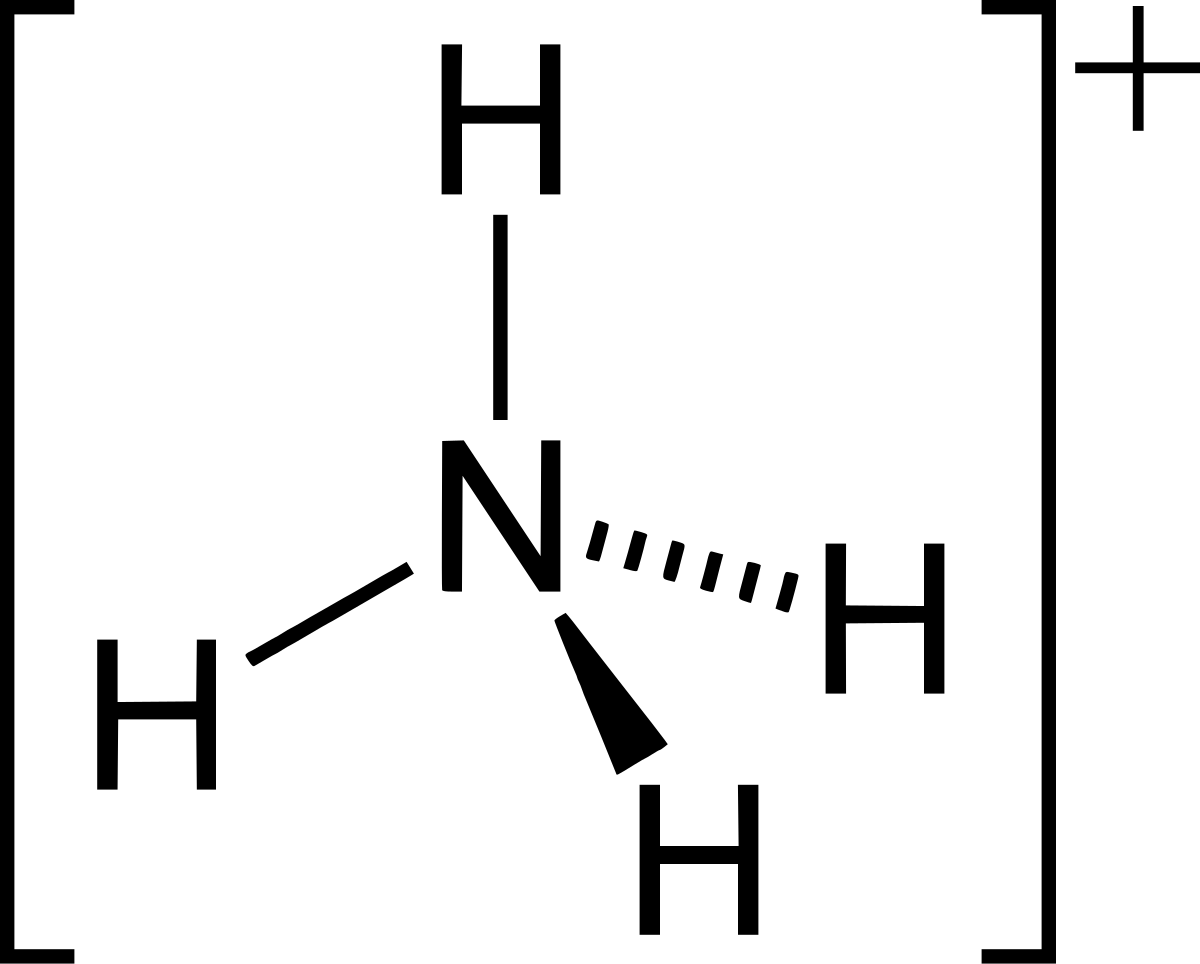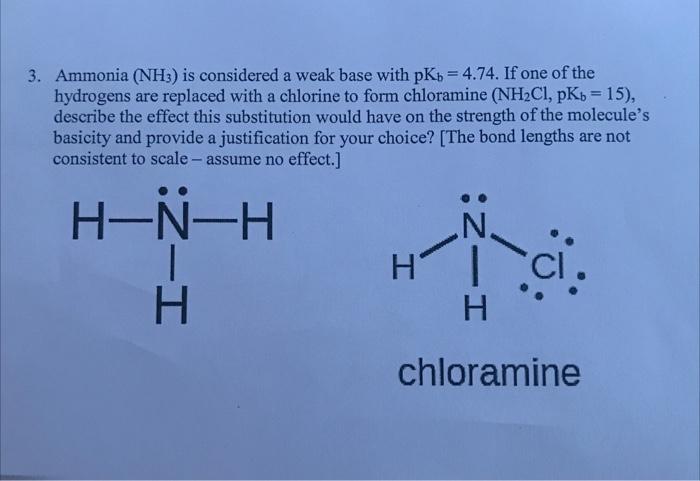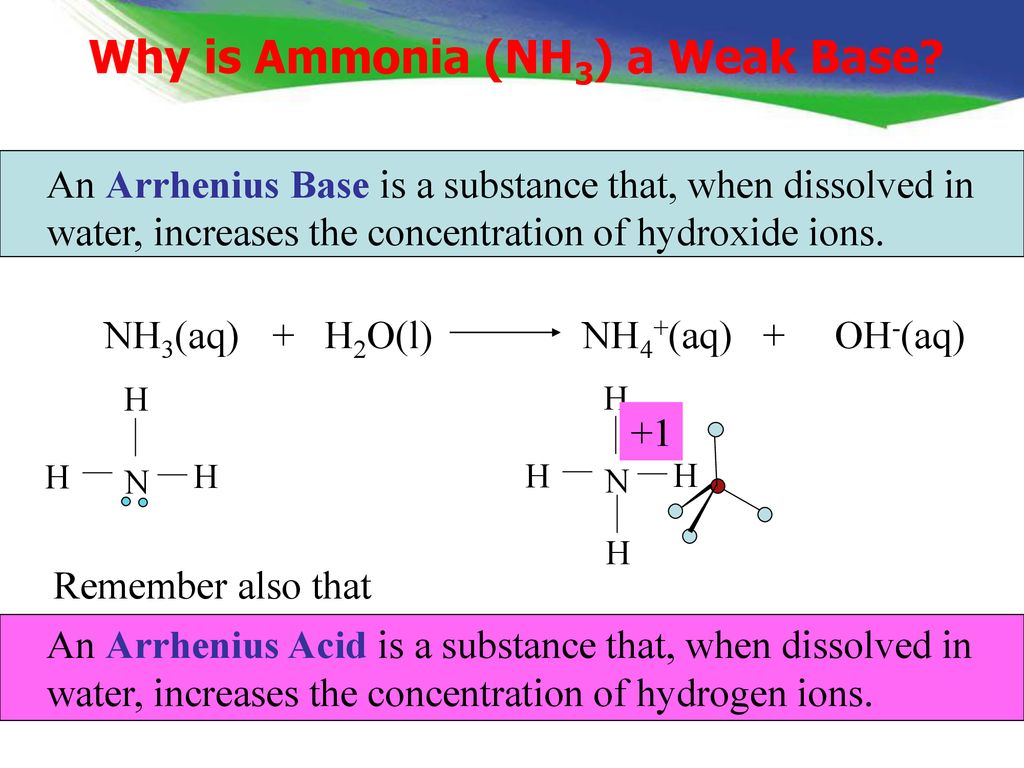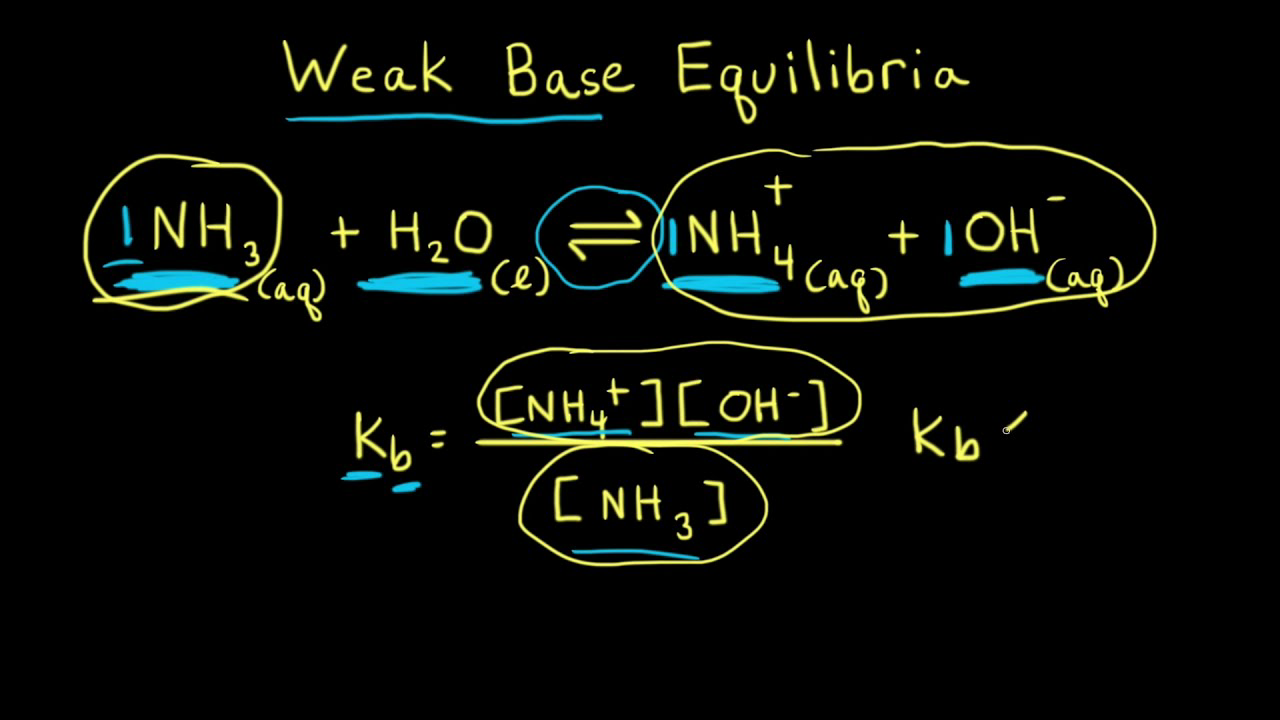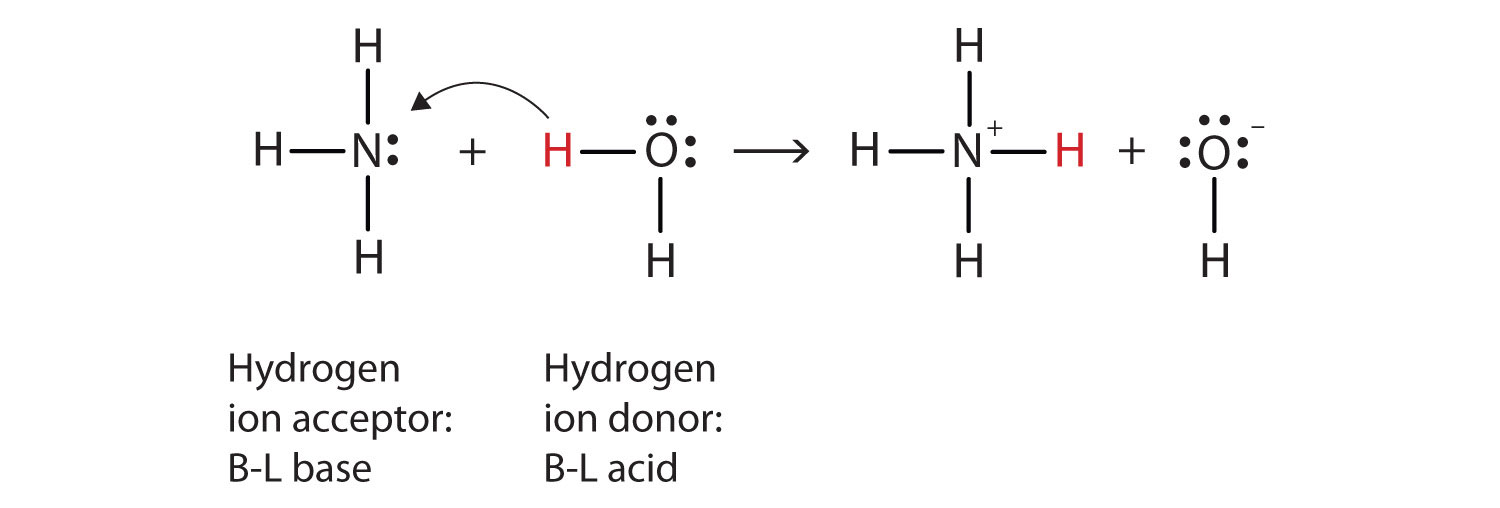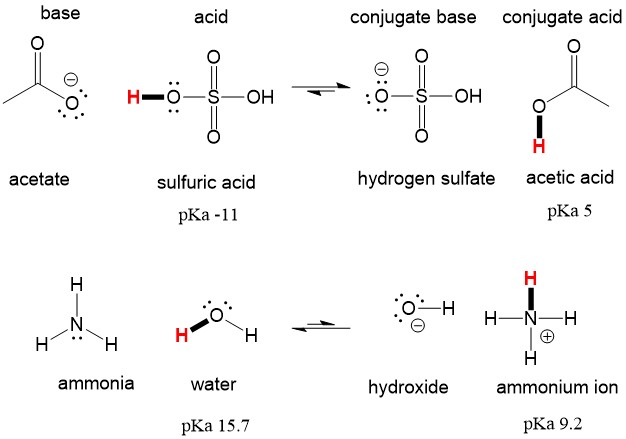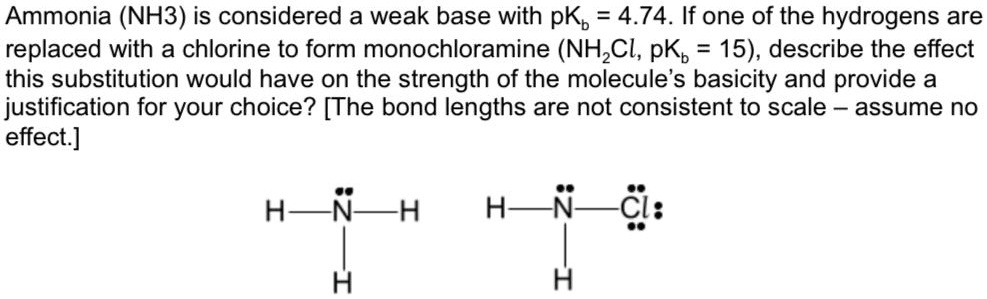
SOLVED: Ammonia (NH3) is considered a weak base with pKb = 4.74. If one of the hydrogens are replaced with a chlorine to form monochloramine (NHZCl, pKb 15) , describe the effect

Manganese‐Catalyzed Ammonia Oxidation into Dinitrogen under Chemical or Electrochemical Conditions** - Toda - 2021 - ChemPlusChem - Wiley Online Library