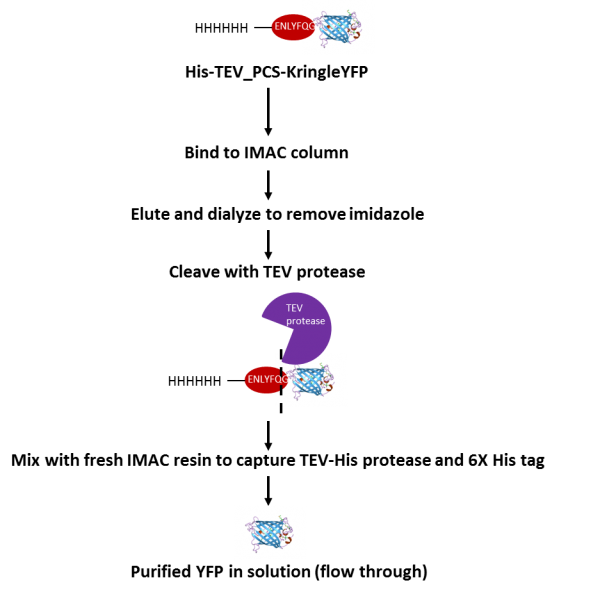
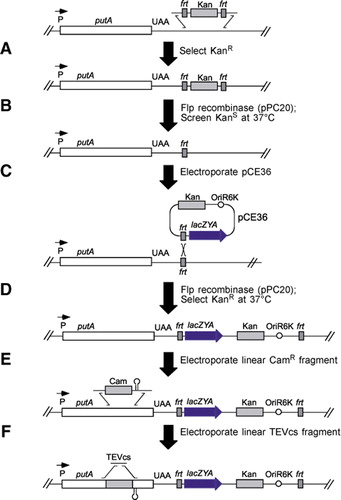
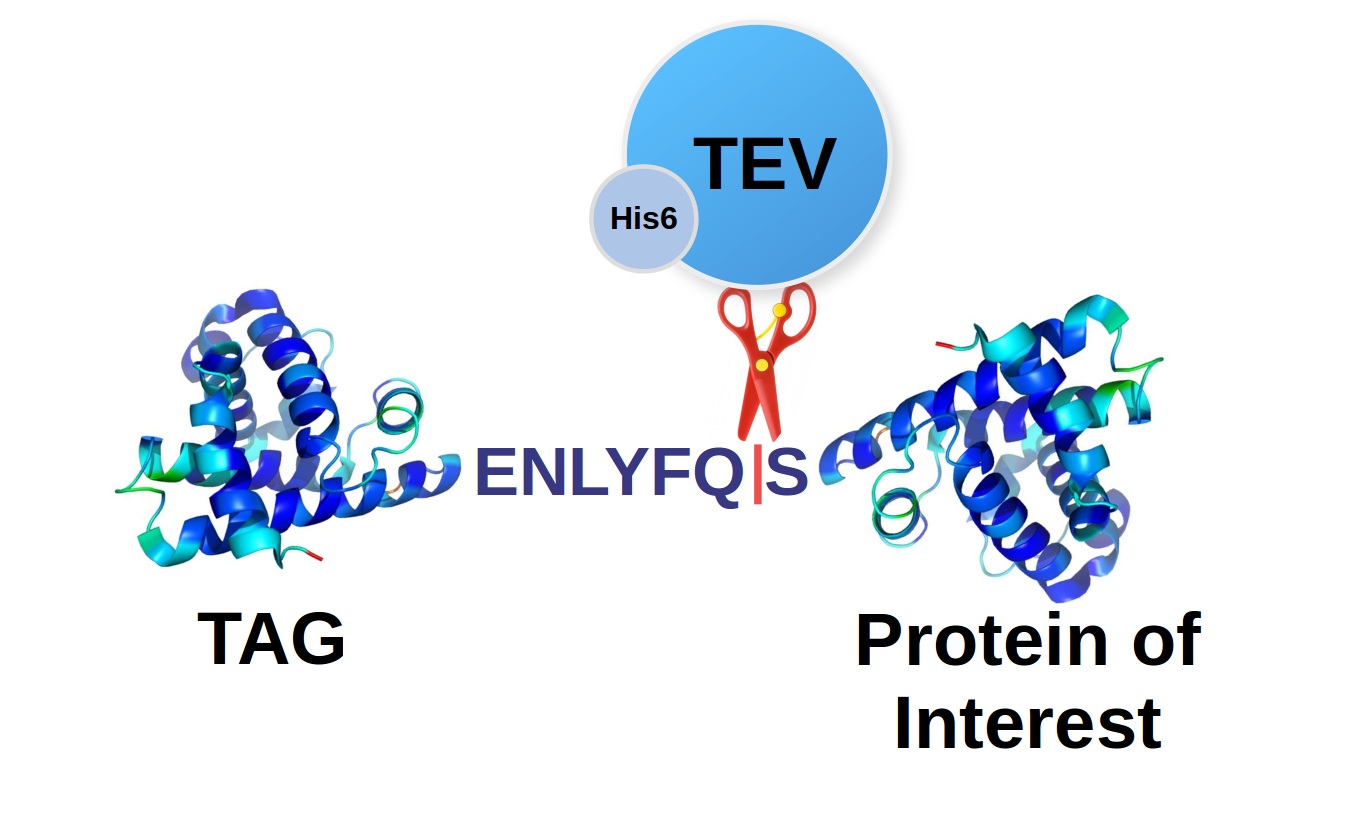
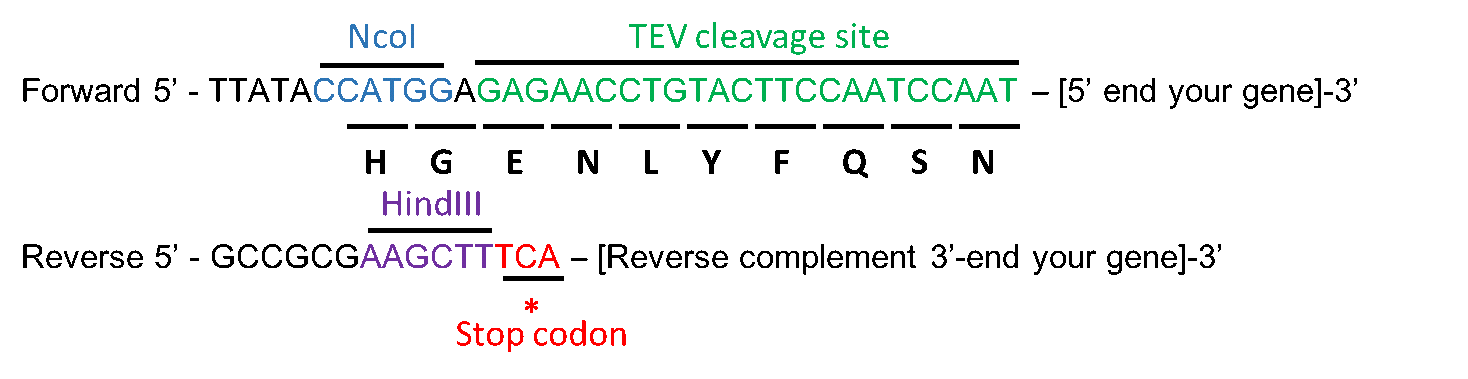
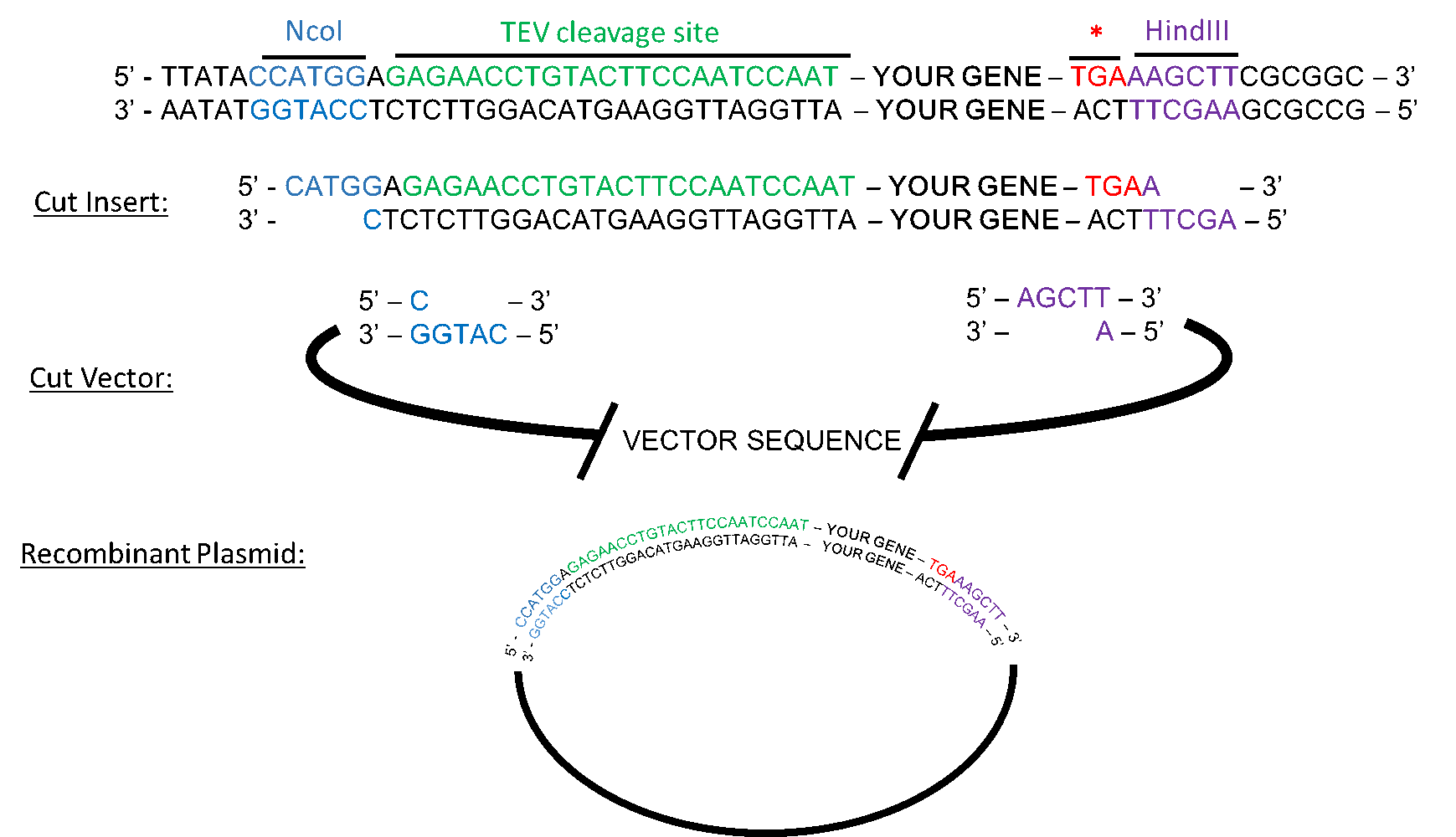
Tobacco Etch Virus (TEV) protease protein fusions and immobilization... | Download Scientific Diagram
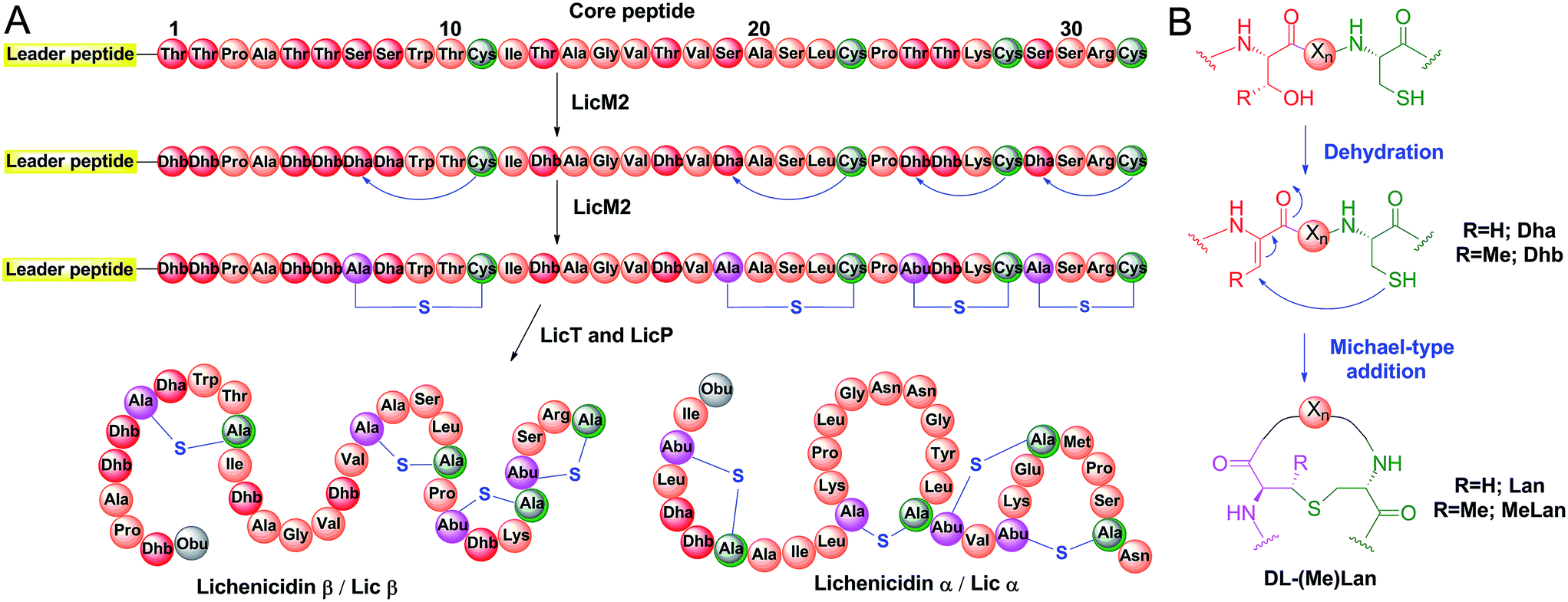
Applications of the class II lanthipeptide protease LicP for sequence-specific, traceless peptide bond cleavage - Chemical Science (RSC Publishing) DOI:10.1039/C5SC02329G

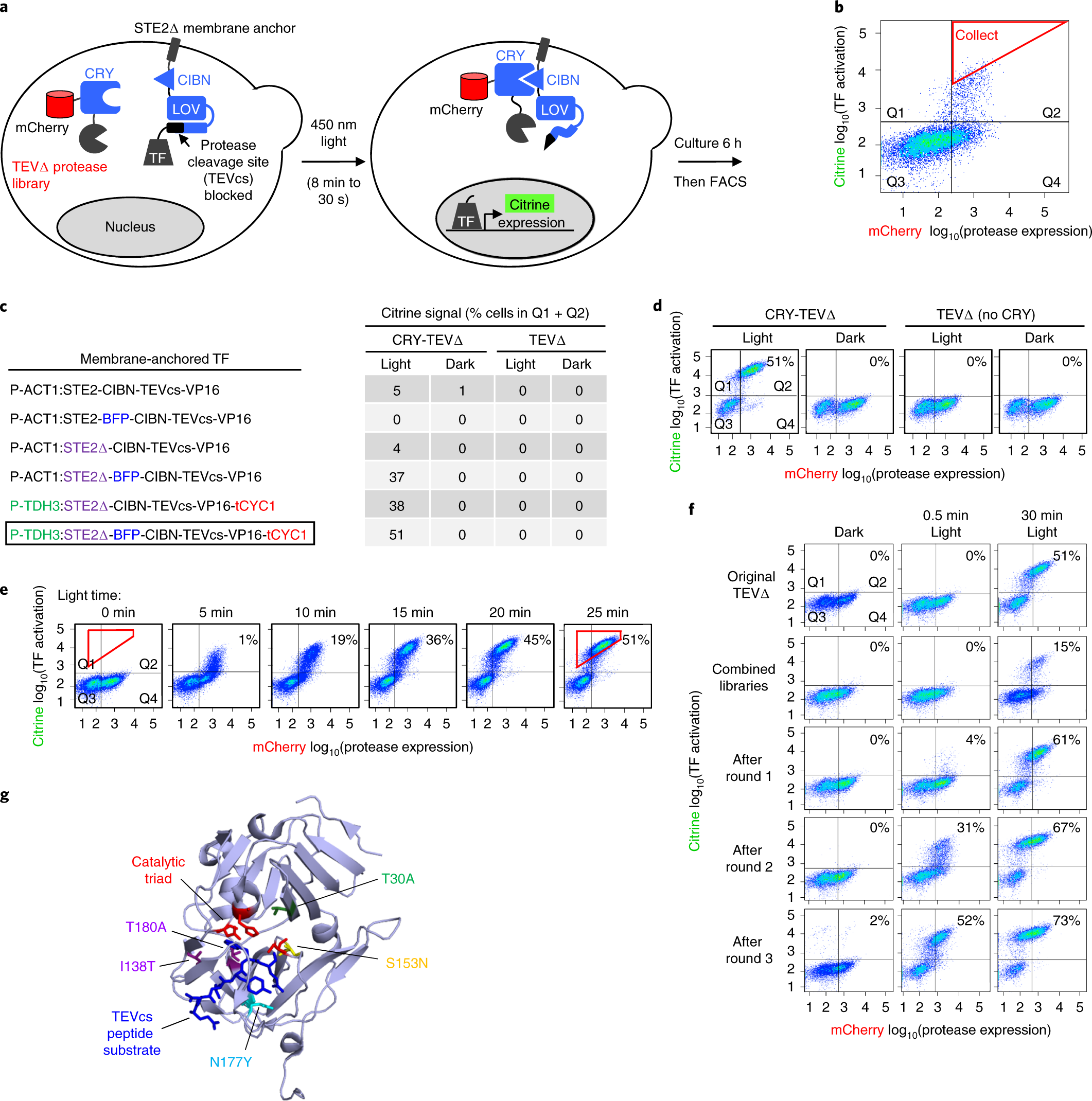
YESS 2.0, a Tunable Platform for Enzyme Evolution, Yields Highly Active TEV Protease Variants | ACS Synthetic Biology

Three-Amino Acid Spacing of Presenilin Endoproteolysis Suggests a General Stepwise Cleavage of γ-Secretase-Mediated Intramembrane Proteolysis | Journal of Neuroscience
Recombinant Production of the Amino Terminal Cytoplasmic Region of Dengue Virus Non-Structural Protein 4A for Structural Studies | PLOS ONE