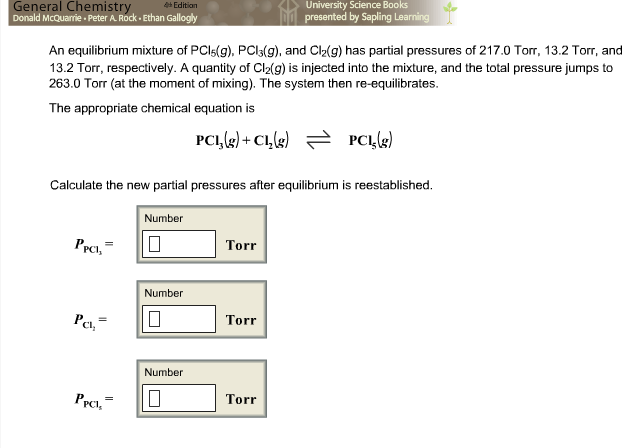
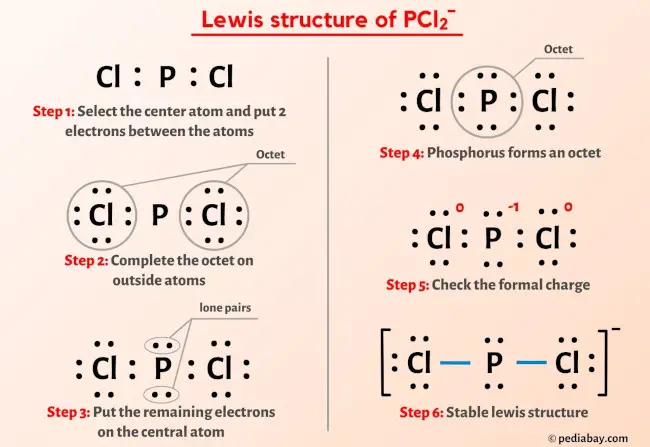
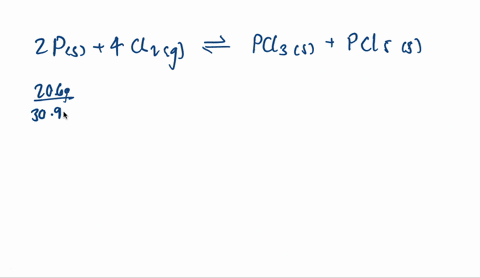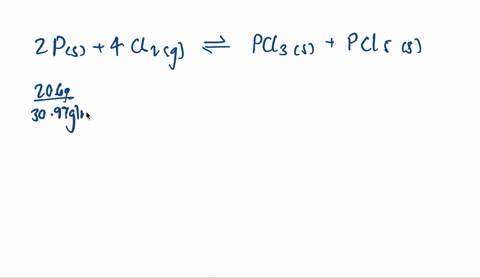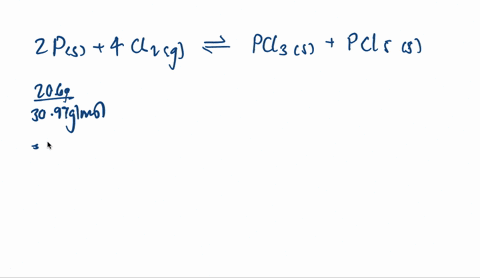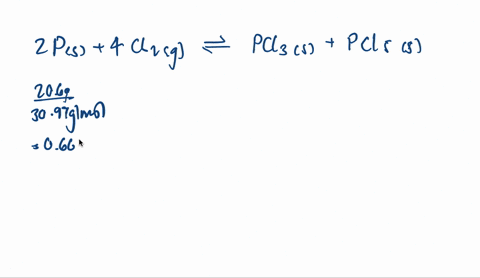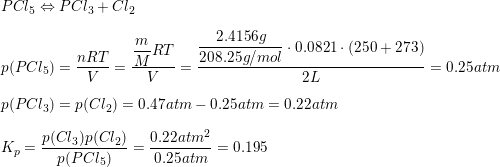Model systems for a stepwise hydrolysis of (a) PCl3, (b) P(OH)Cl2, (c)... | Download Scientific Diagram

At certain temperature(T) for the gas phase reaction: 2H2O(g) + 2Cl2(g) 4HCl(g) + O2(g) , Kp = 12 × 10^8 atm. If Cl2, HCI & O2 are mixed in such a manner

Gaseous NOCl is placed in a closed container at 173 oC, where it partially decomposes to NO and Cl2:2 NOCl(g) 2 NO(g) + 1 Cl2(g) At equilibrium it is found that p(NOCl) =
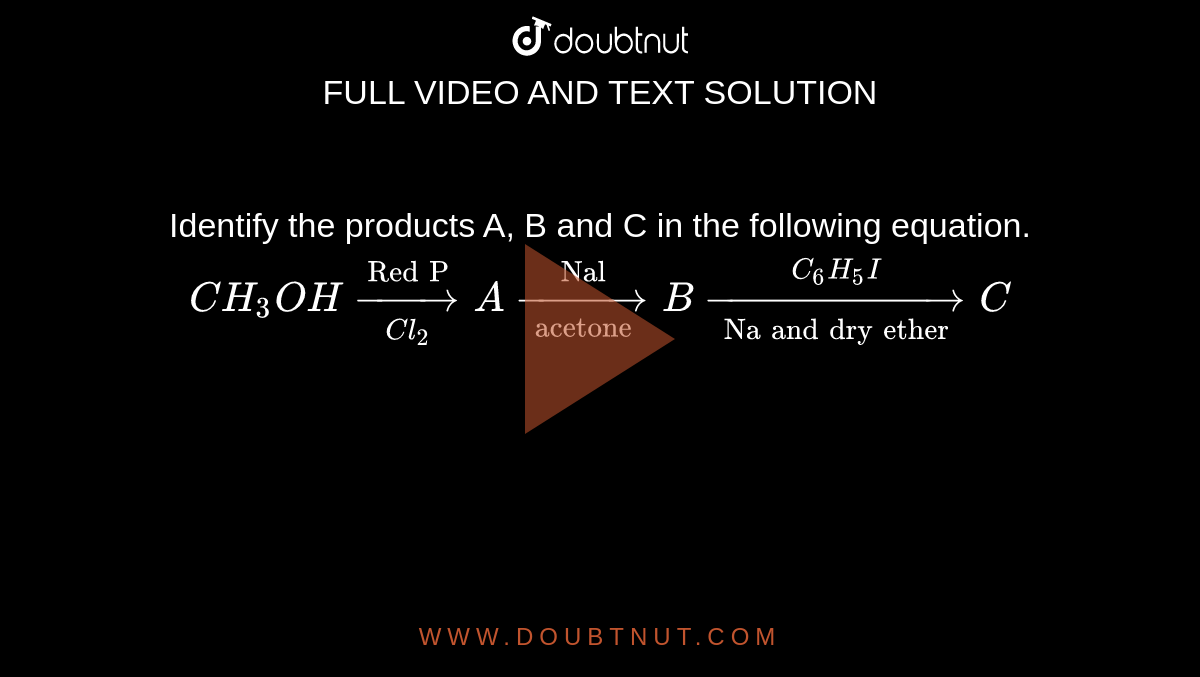
CH3CH2 COOH,+Cl2(red P)→ A ,(alc.KOH) → B. What is B? - Sarthaks eConnect | Largest Online Education Community
a' mol of PCL5 gives PCl3 and Cl2 the mole fraction of PCl3 at equilibrium is 0.25 and the totol pressure is 2 atm. The partial pressure of Cl2 at equilibrium is
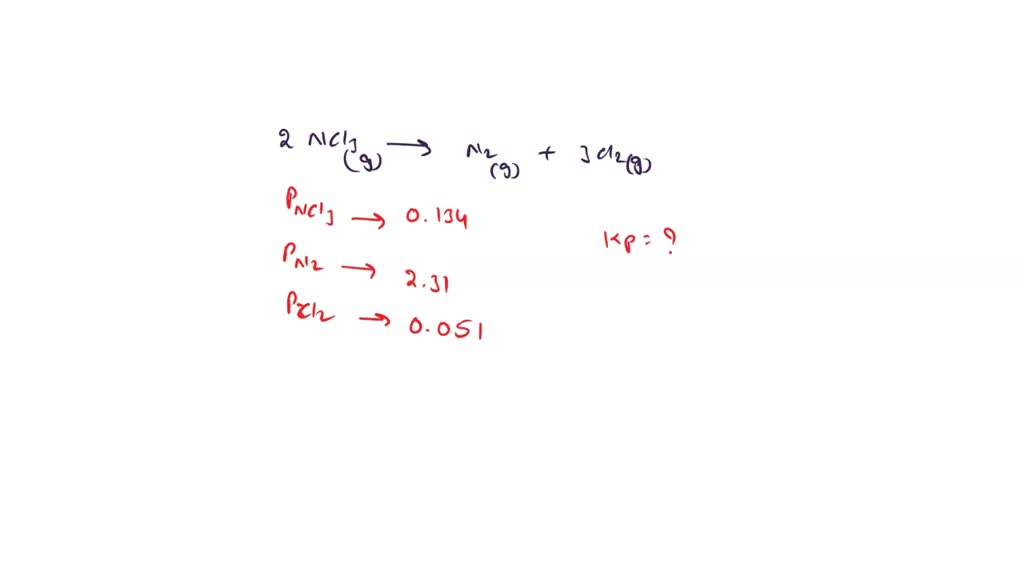
SOLVED: For the reaction 2NCl3(g) ↔ N2(g) + 3Cl2(g), the equilibrium pressures are P(NCl3) = 0.134 atm P(N2) = 2.31 atm P(Cl2) = 0.0510 atm Determine Kp for this reaction

OneClass: Chem 401 Discussion workshop #5 Name 3. Initially, ph-pCl2=0.10 atm plci-0 a) Set up expres...
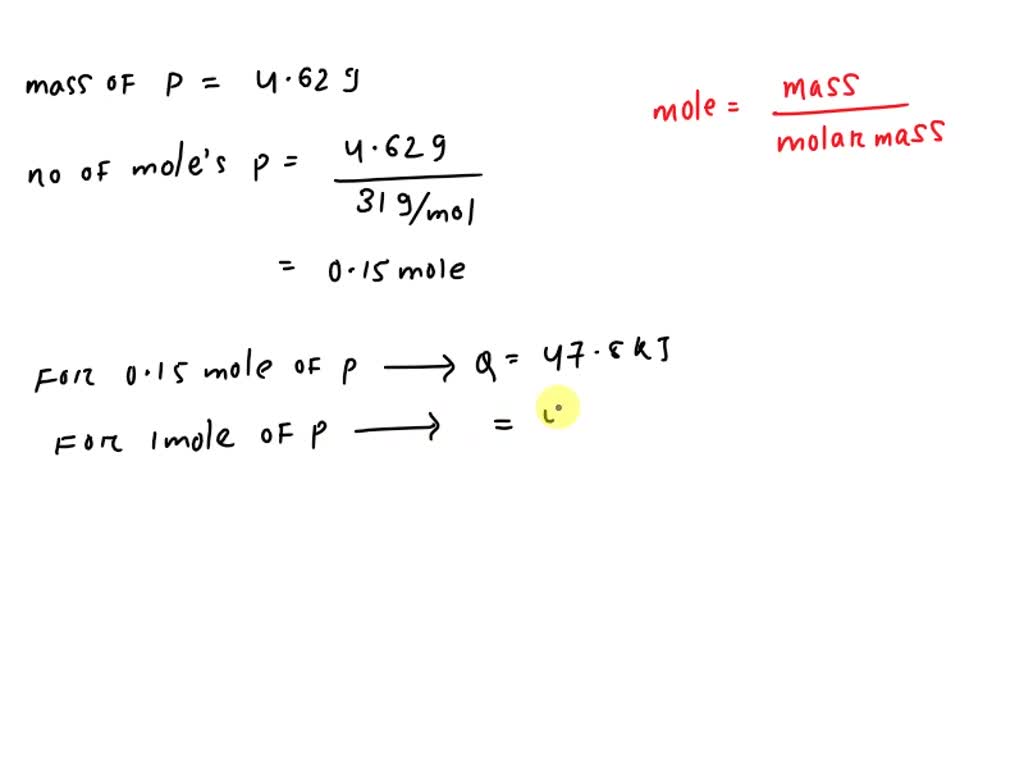
SOLVED: When solid phosphorus, P, reacts with gaseous chlorine, Cl2, it forms liquid phosphorus trichloride, PCl3. When 4.62 grams of phosphorus reacts with excess chlorine at constant pressure 47.8 kJ of heat