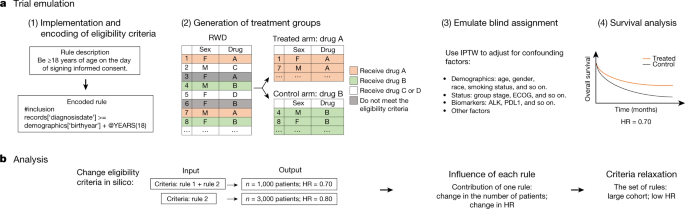
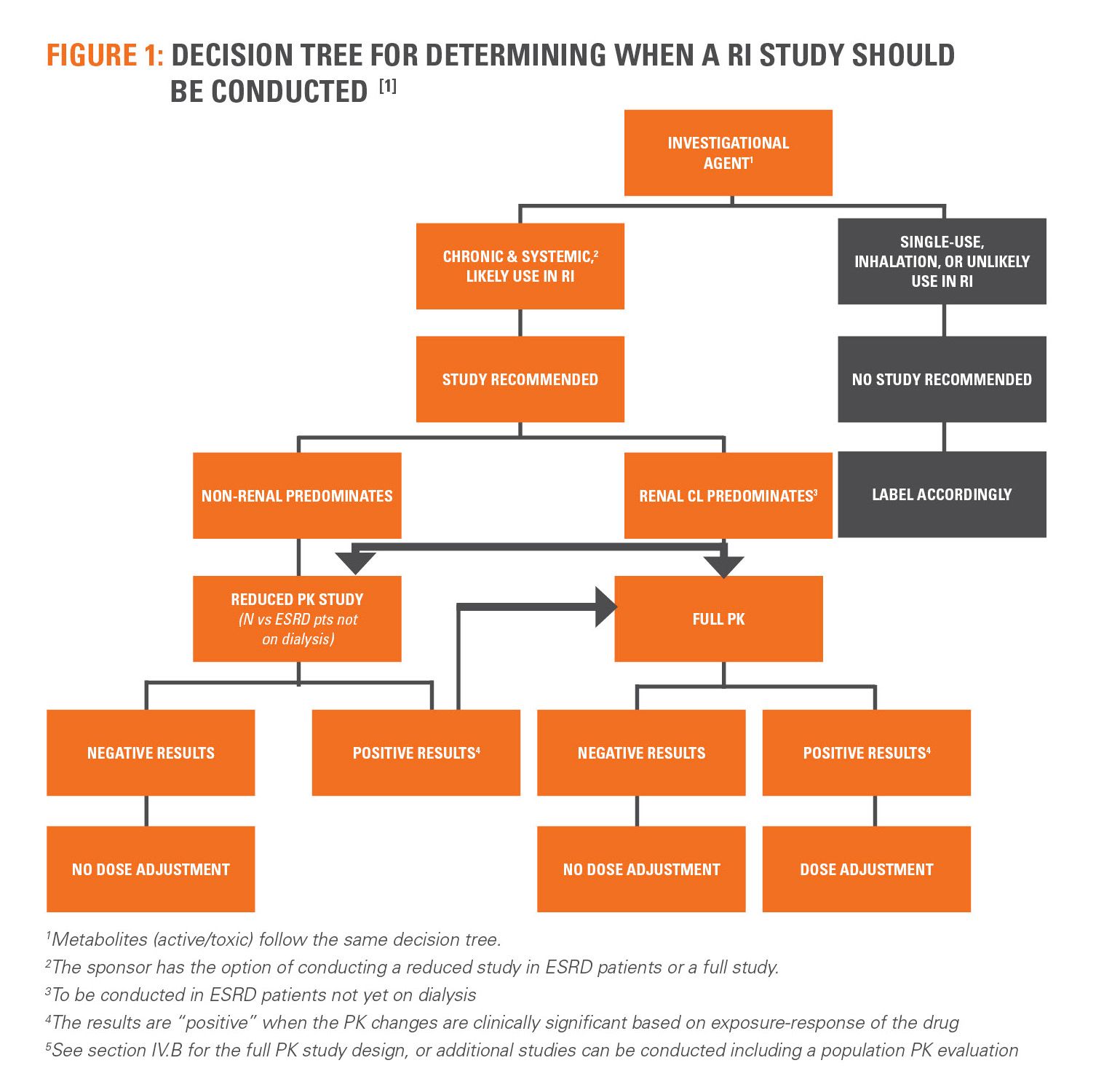
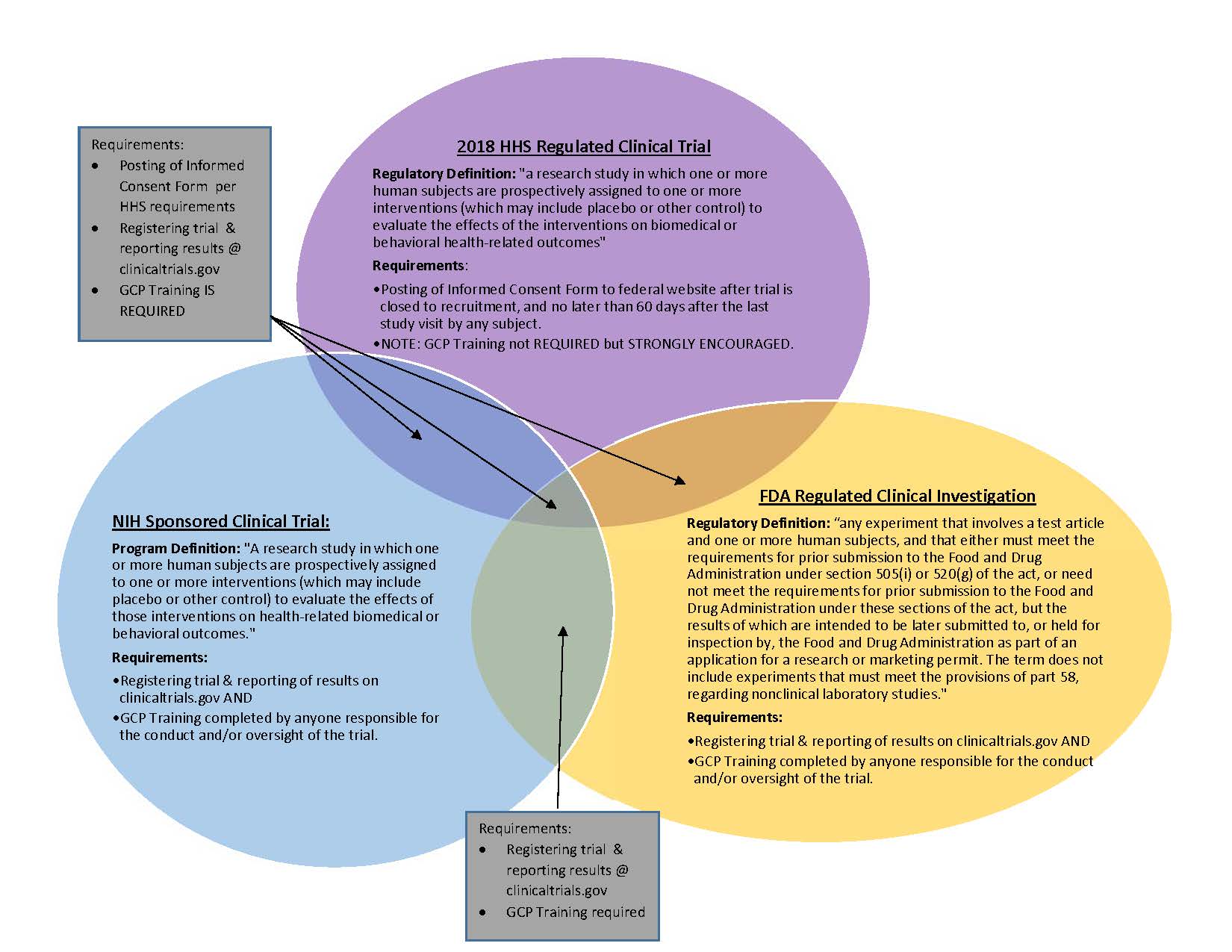
Completeness of Reporting of Patient-Relevant Clinical Trial Outcomes: Comparison of Unpublished Clinical Study Reports with Publicly Available Data | PLOS Medicine

Pragmatic Design of Randomized Clinical Trials for Heart Failure: Rationale and Design of the TRANSFORM-HF Trial - ScienceDirect
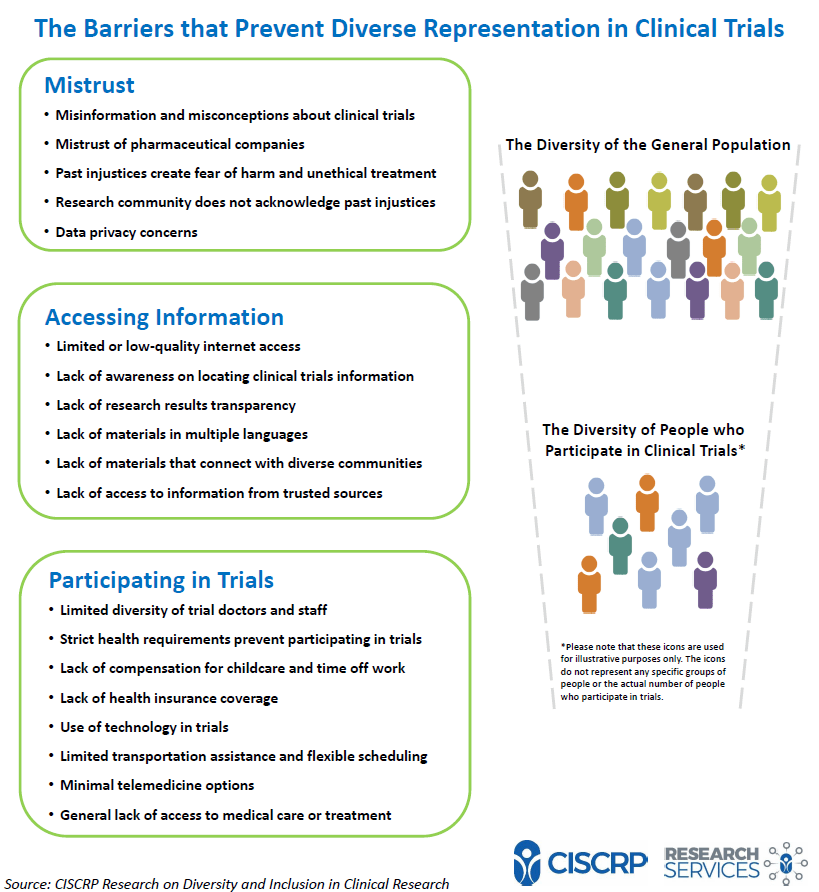
Doing Our Part: Improving Diversity in Clinical Research Participation - Center for Information & Study on Clinical Research Participation

What, how, when and who of trial results summaries for trial participants: stakeholder-informed guidance from the RECAP project | BMJ Open

Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study - The Lancet

Publication and reporting of clinical trial results: cross sectional analysis across academic medical centers | The BMJ

The Ontology of Clinical Research (OCRe): An informatics foundation for the science of clinical research - ScienceDirect