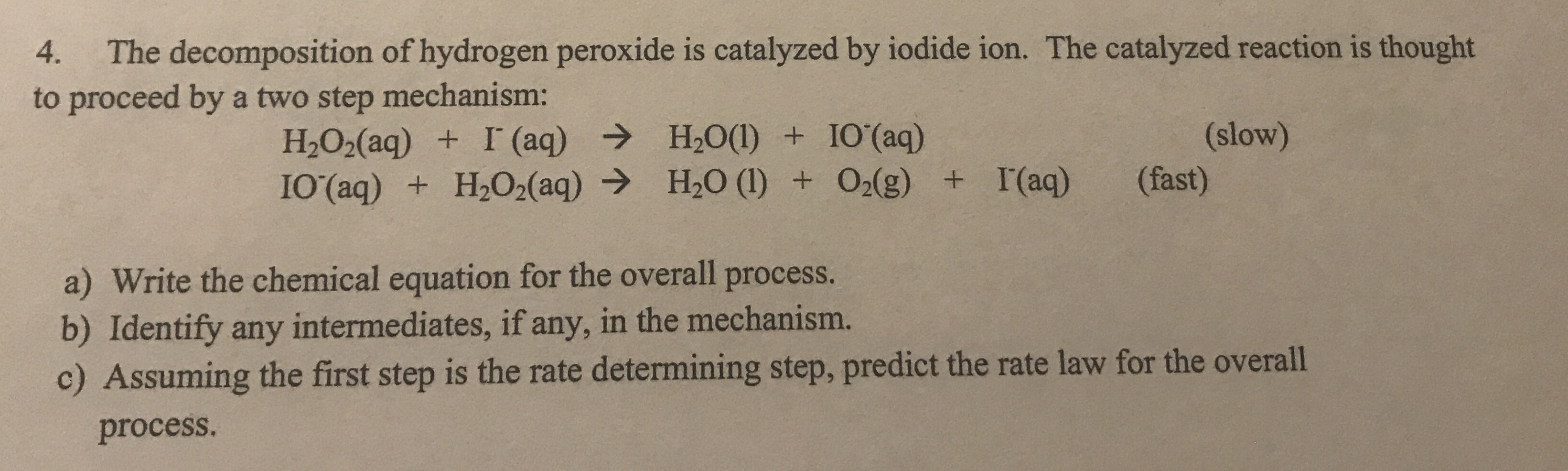
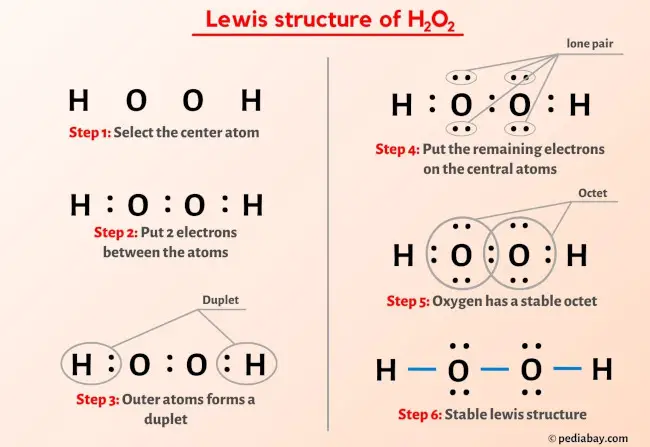
What is the balanced half-reaction equation for H2O2 (aq) acting as a reducing agent in an acidic aqueous solution? - Quora

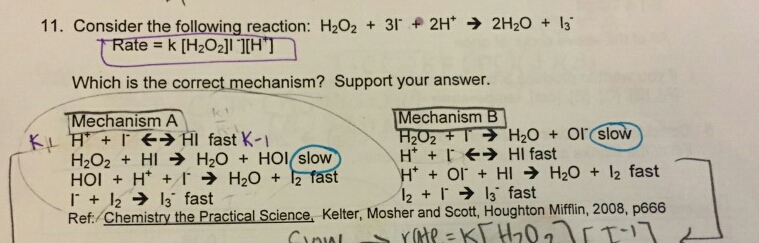
SOLVED: Consider this balanced chemical equation: H2O2 (aq)+3I−(aq)+2H+(aq)→I3−(aq)+2H2O(l)H2O2(aq)+3I−(aq)+2H+(aq)→I3−(aq)+2H2O(l) In the first 10.0 seconds of the reaction, the concentration of I−I− drops from 1.240 MM to 0.861 MM. Determine ...
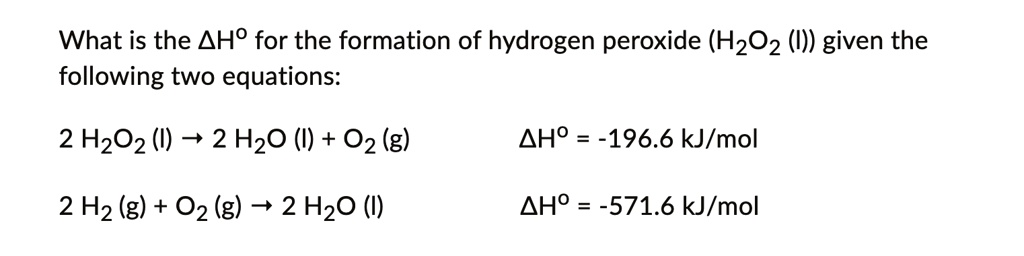
SOLVED: What is the AHO for the formation of hydrogen peroxide (H2O2 (I)) given the following two equations: 2 H2O2 () 2 HzO () +02 (g) AHo -196.6 kJ/mol 2 Hz (g) + 02 (g) 2 Hz0 () AHO = -571.6 kJ/mol

The decomposition of H2O2 has a strong thermodynamic driving force 2H2O2→ 2H2O + O2(g) Δ H = - 99kj/mole,Δ s = + 69JK^-1 mole^-1 Addition of solution of KI causes H2O2 to

The Role of Bicarbonate-Based Electrolytes in H2O2 Production through Two-Electron Water Oxidation | ACS Energy Letters
What is the balanced half-reaction equation for H2O2 (aq) acting as a reducing agent in an acidic aqueous solution? - Quora

Peracetic Acid Oxidation of Saline Waters in the Absence and Presence of H2O2: Secondary Oxidant and Disinfection Byproduct Formation | Environmental Science & Technology
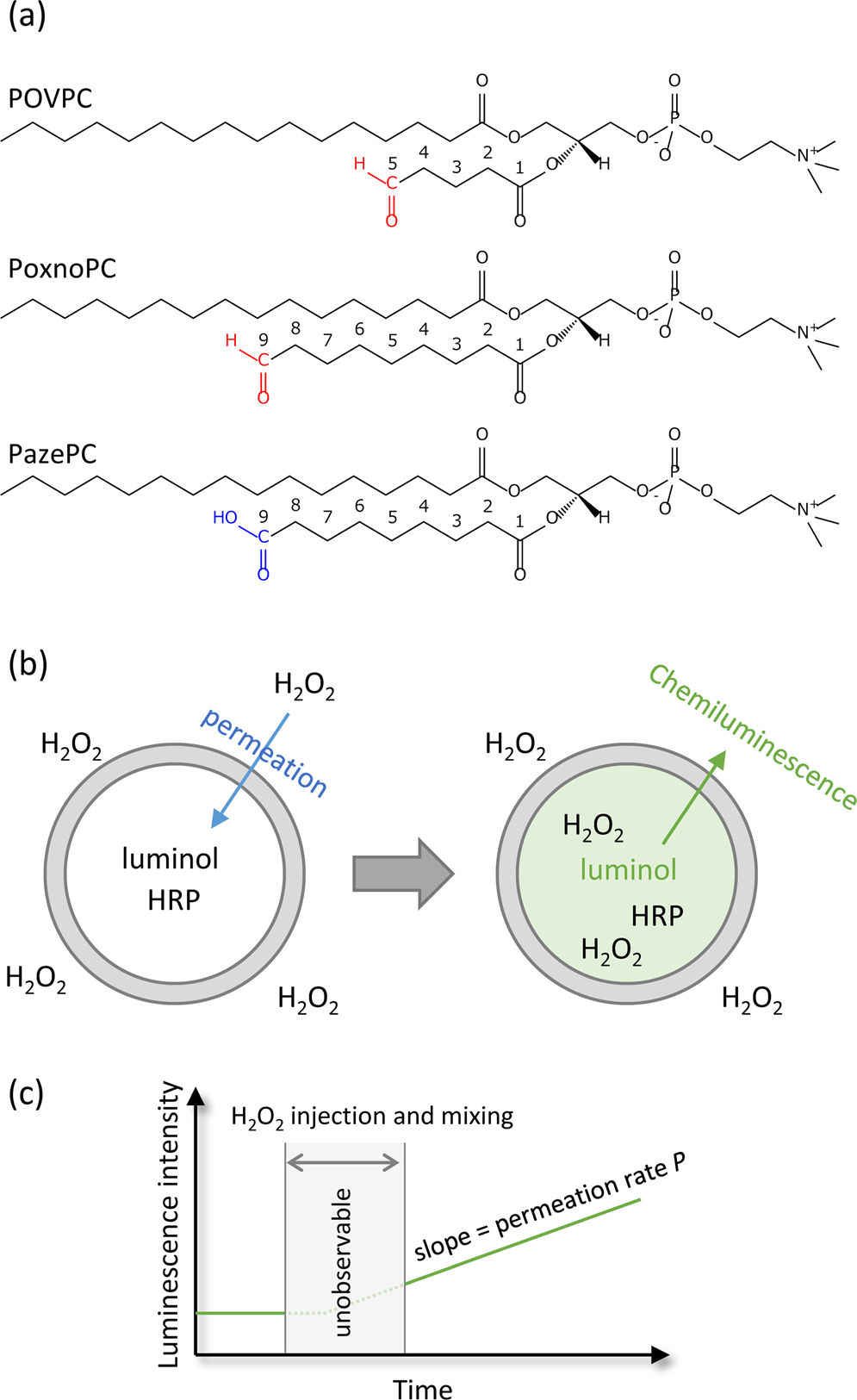
Role of Oxidized Lipids in Permeation of H2O2 Through a Lipid Membrane: Molecular Mechanism of an Inhibitor to Promoter Switch | Scientific Reports

Given below are the two reactions of H2O2 . Mark the correct statement which follows :(i) 2KMnO4 + 3H2SO4 + 5H2O2→ K2SO4 + 2MnSO4 + 8H2O + 5O2 (ii) 2Cr(OH)3 + 4NaOH + 3H2O2→ 2Na2CrO4 + 8H2O
I) H2O2 + O3 → H2O + 2O2 (II) H2O2 + Ag2O → 2Ag + H2O + O2 Role of hydrogen peroxide in the - Sarthaks eConnect | Largest Online Education Community

SOLVED: Which represents the overall (net) reaction of this mechanism: step 1: H2O2 + I- → H2O + OI- step 2: H2O2 + OI- → H2O + O2 + I- Note that



![Integrated rate laws ln[A] = -kt + ln[A]0 rate = k[A] - ppt download Integrated rate laws ln[A] = -kt + ln[A]0 rate = k[A] - ppt download](https://slideplayer.com/slide/14728131/90/images/4/Reaction+mechanism+2H2O2+%28aq%29+%EF%82%AE+2H2O%28l%29+%2B+O2%28g%29+rate+%3D+k%5BH2O2%5D+%5BI-%5D.jpg)