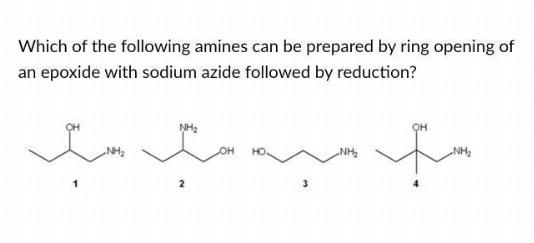
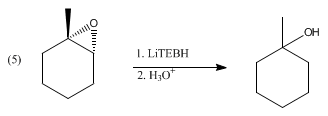Catalytic reductive ring opening of epoxides enabled by zirconocene and photoredox catalysis - ScienceDirect

Synthesis of α-Alkylated Ketones via Selective Epoxide Opening/Alkylation Reactions with Primary Alcohols | Organic Letters

A New and Efficient Epoxide Ring Opening via Poor Nucleophiles: Indole, p-Nitroaniline, Borane and O-Trimethylsilylhydroxylamine in Lithium Perchlorate

Catalytic Reduction of Cyclic Ethers with Hydrosilanes - Park - 2019 - Chemistry – An Asian Journal - Wiley Online Library

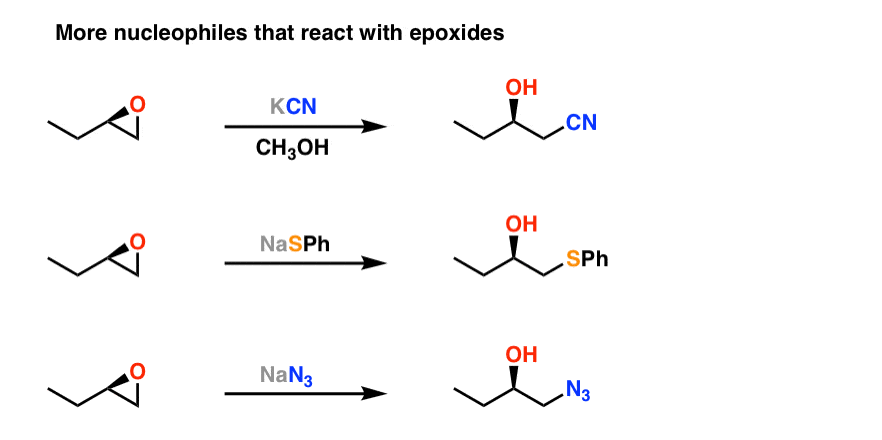
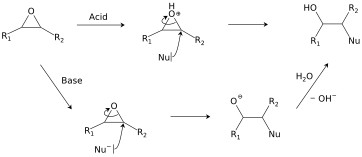
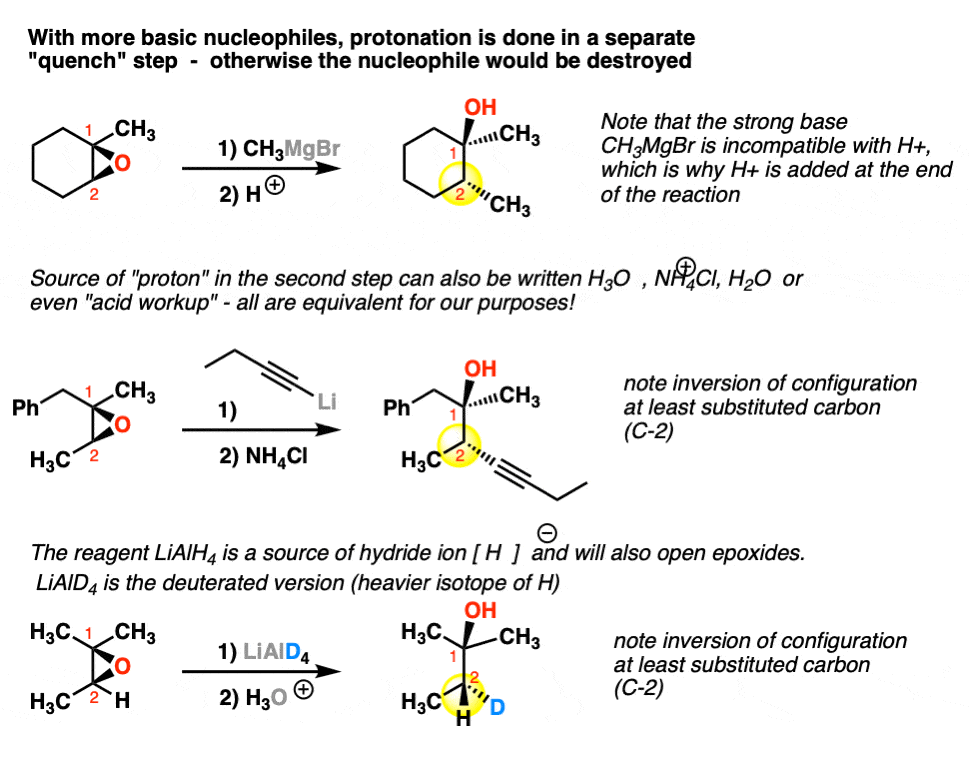
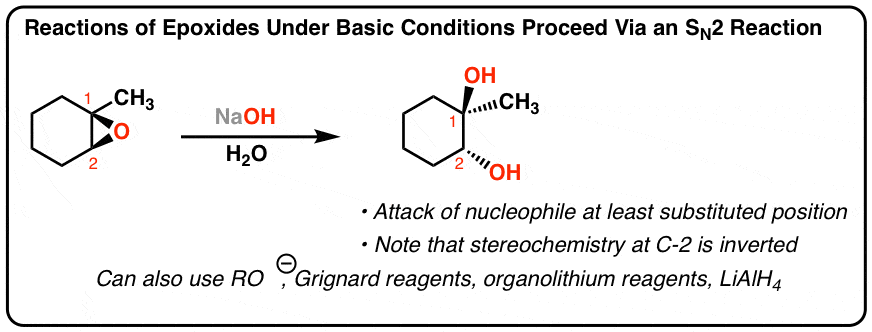
This explanation says that there is an inversion in stereochemistry when the reducing agent reduces the epoxide, but the stereochemistry after reduction stays the same. Can anyone explain? : r/OrganicChemistry
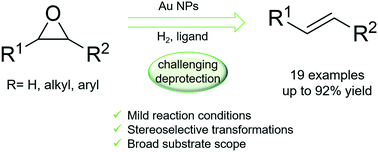
Clean protocol for deoxygenation of epoxides to alkenes via catalytic hydrogenation using gold - Catalysis Science & Technology (RSC Publishing)

Catalytic Hydrogenation of Epoxides to Alcohols - Thiyagarajan - 2022 - Chemistry – An Asian Journal - Wiley Online Library

Epoxides are reduced by treatment with lithium aluminum hydride to yield alcohols. Propose a mechanism for this reaction. | Homework.Study.com
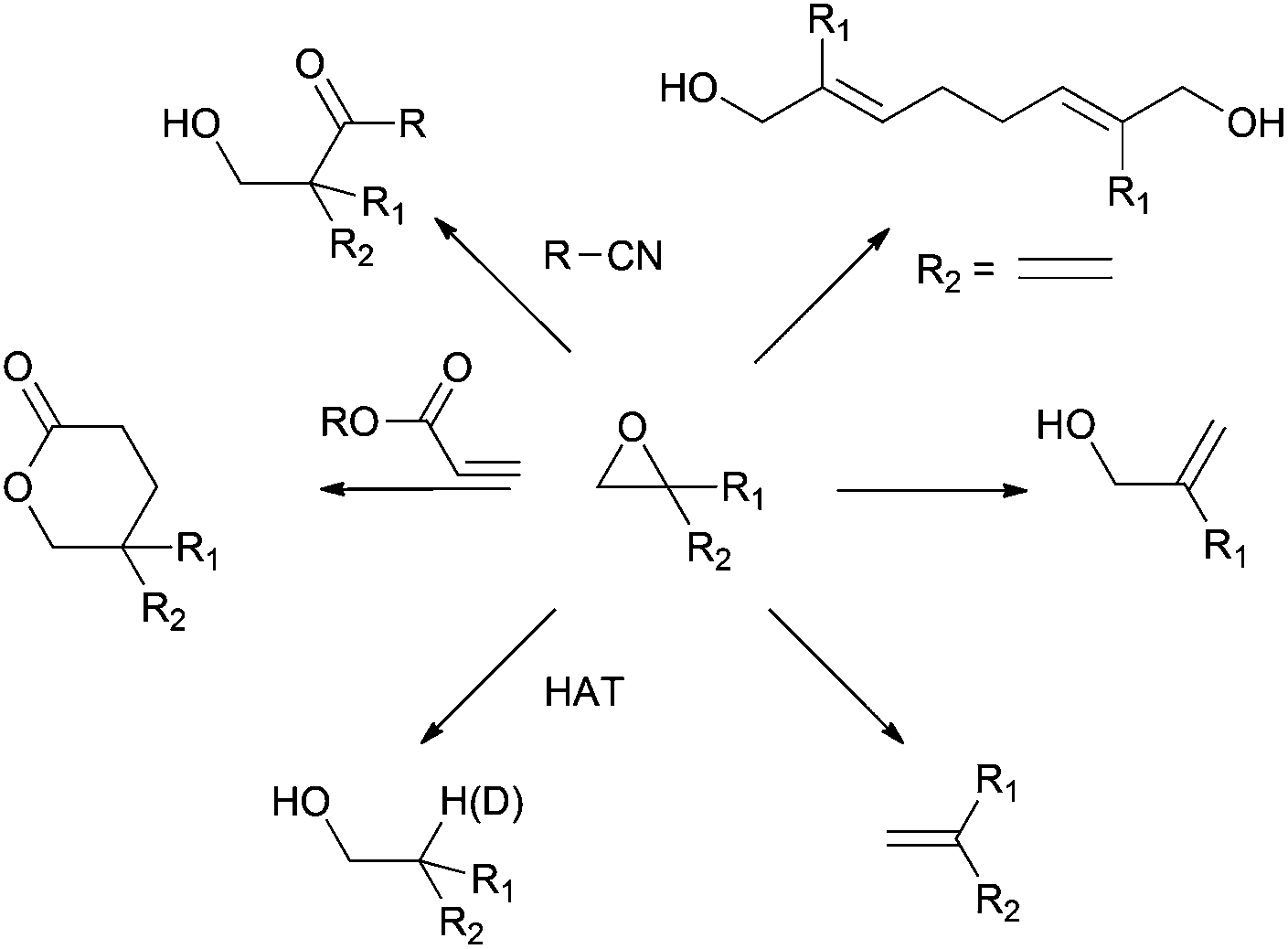
Recent applications of Cp 2 TiCl in natural product synthesis - Organic Chemistry Frontiers (RSC Publishing) DOI:10.1039/C3QO00024A