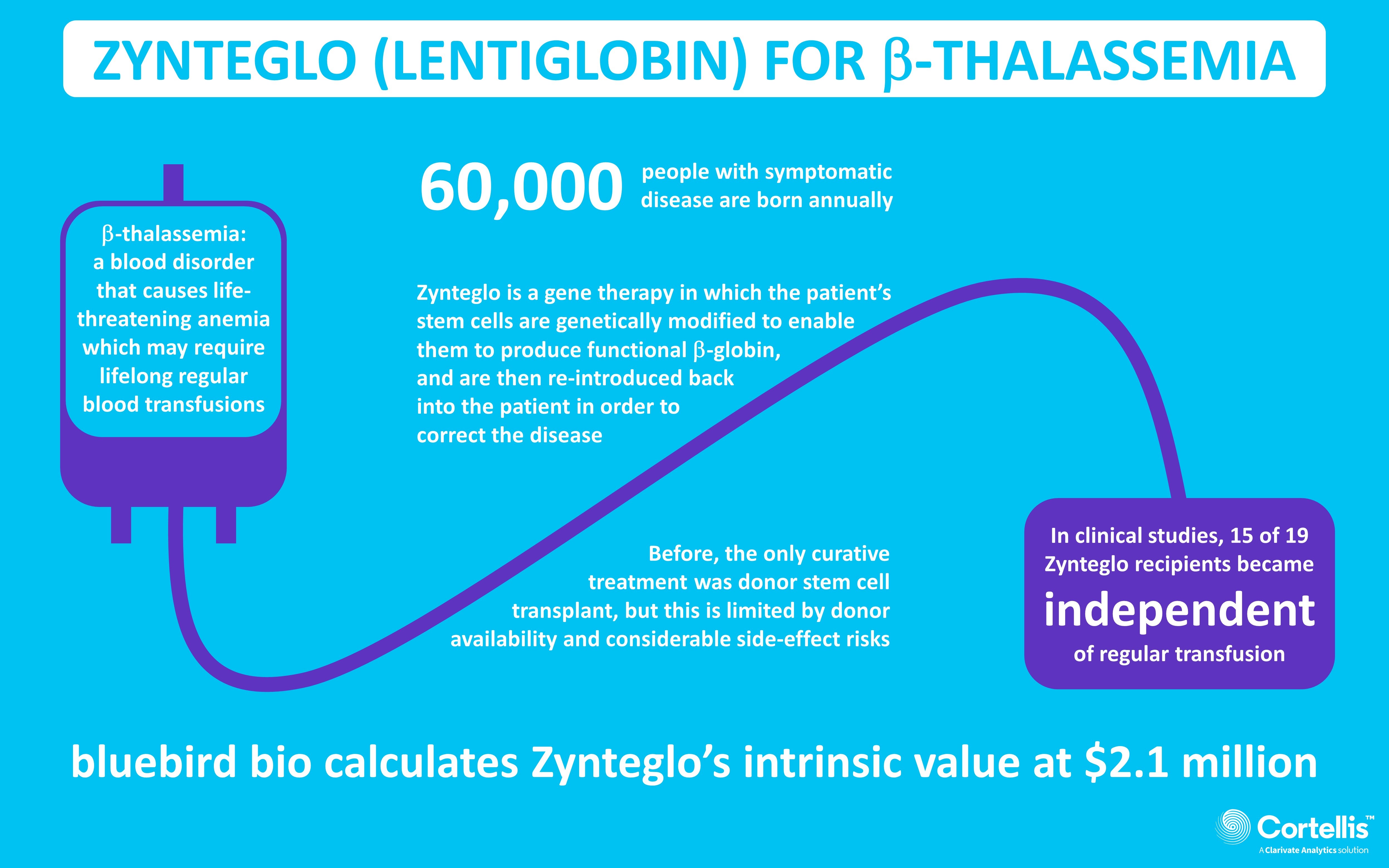
ICER's Favorable Assessment Of Bluebird Bio's Gene Therapy Zynteglo May Have Important Pricing And Reimbursement Implications
Bluebird bio's gene therapy Zynteglo could have high price tag defense in beta thalassemia - Pharmaceutical Technology

bluebird bio Announces FDA Approval of ZYNTEGLO®, the First Gene Therapy for People with Beta-Thalassemia Who Require Regular Red Blood Cell Transfusions - bluebird bio, Inc.

Bluebird Bio To Resume Zynteglo Gene Therapy Marketing In Europe After Positive Recommendation From PRAC
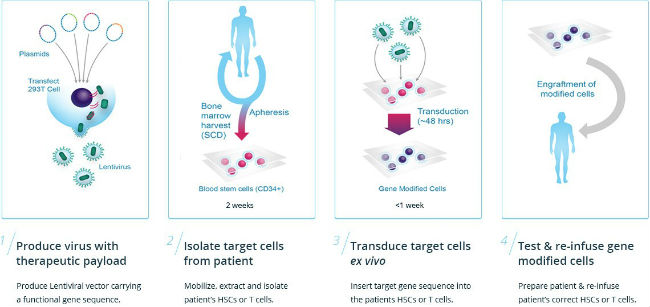
bluebird bio Launches Gene Therapy for Severe Blood Disorder in Germany | North Carolina Biotechnology Center

FDA approves bluebird bio's Zynteglo gene therapy for beta thalassemia; shares up 7% | Seeking Alpha

bluebird bio Announces EU Conditional Marketing Authorization for ZYNTEGLO™ (autologous CD34+ cells encoding βA-T87Q-globin gene) Gene Therapy for Patients 12 Years and Older with Transfusion-Dependent β-Thalassemia Who Do Not Have β0/β0 Genotype

bluebird bio Announces EU Conditional Marketing Authorization for ZYNTEGLO™ (autologous CD34+ cells encoding βA-T87Q-globin gene) Gene Therapy for Patients 12 Years and Older with Transfusion-Dependent β-Thalassemia Who Do Not Have β0/β0 Genotype

bluebird bio wins back-to-back landmark FDA approvals for first-in-class gene therapies - Pharmaceutical Technology

FDA Approves bluebird bio's ZYNTEGLO®, the First Gene Therapy for People with Transfusion-Dependent Beta-Thalassemia - The Cooley's Anemia Foundation