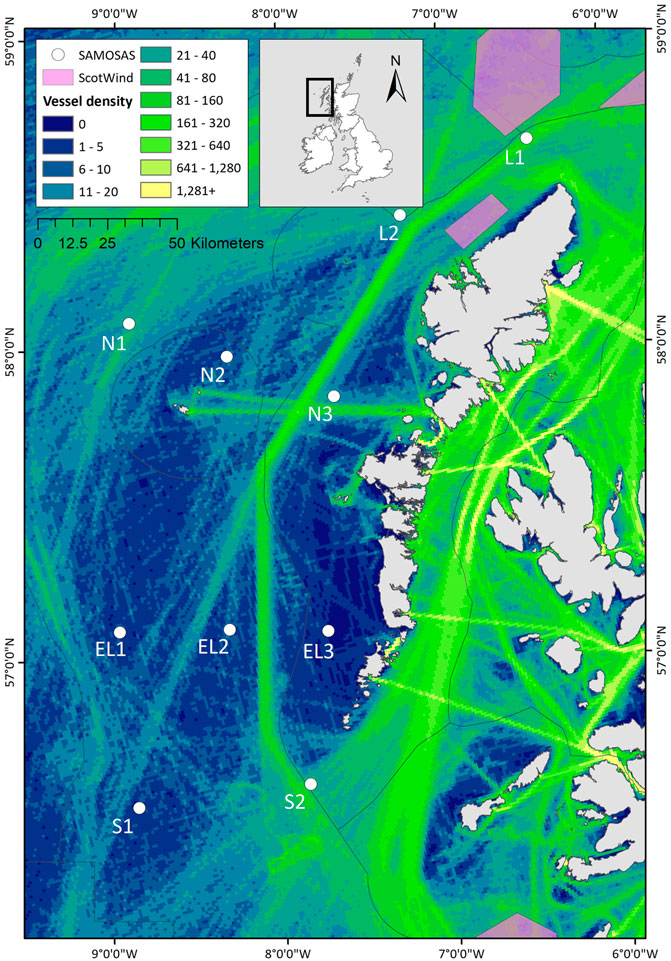
Frontiers | Monitoring cetacean occurrence and variability in ambient sound in Scottish offshore waters
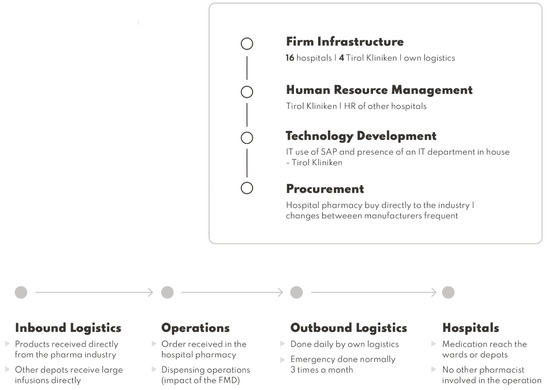
IJERPH | Free Full-Text | Falsified Medicines Directive in a Secondary Care Environment—Impact on Supply Chain
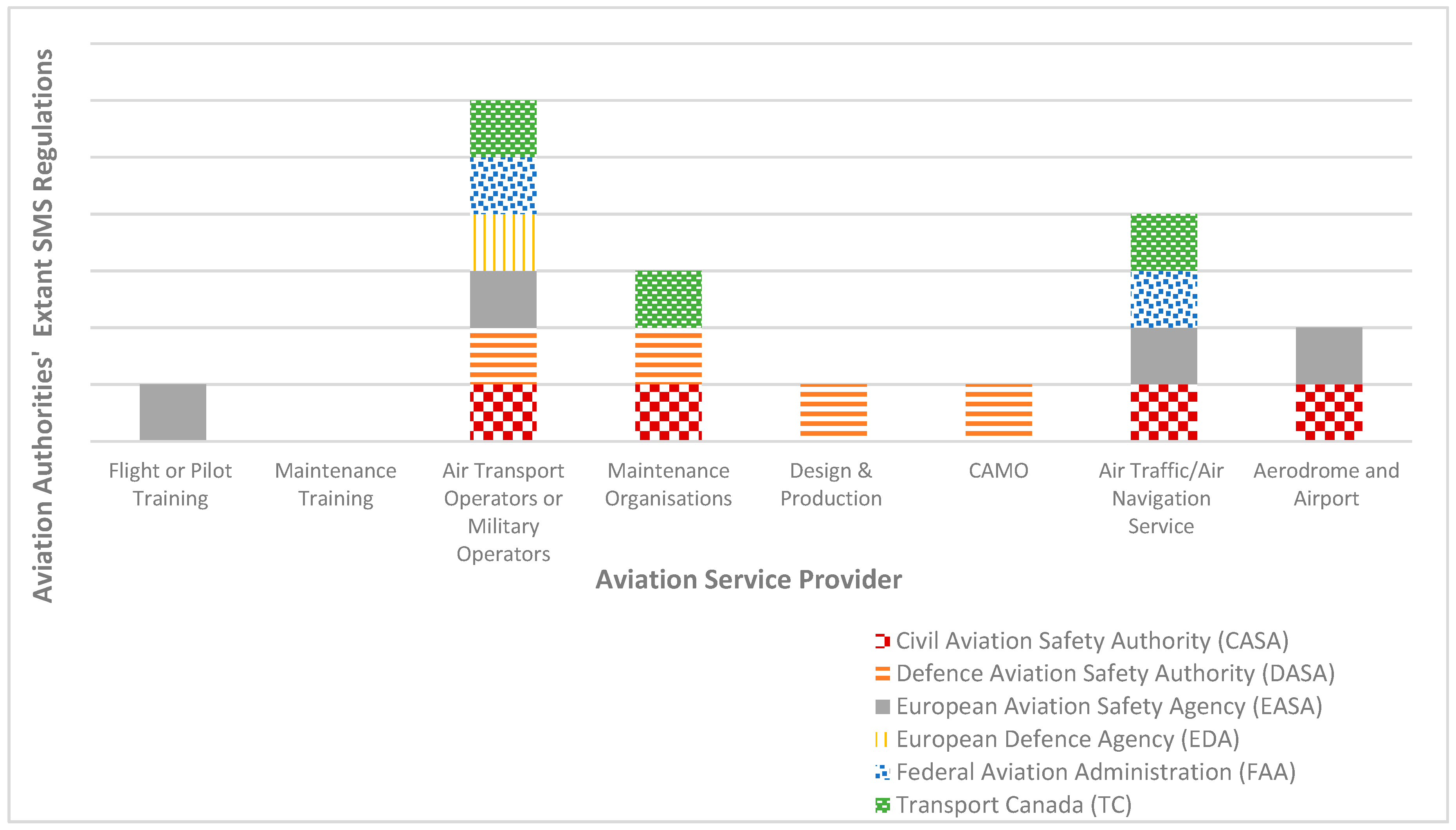
Aerospace | Free Full-Text | The Regulatory Framework for Safety Management Systems in Airworthiness Organisations
AIDE MEMOIRE FOR GDP INSPECTION OF WHOLESALERS COMPLIANCE WITH COMMISSION DELEGATED REGULATION (EU) 2016/161 FOR SAFETY FEATURE
DECISION OF THE EEA JOINT COMMITTEE - No 222 / 2016 - of 2 December 2016 - amending Annex I (Veterinary and p

Regional empowerment through decentralised governance under a centralised regulatory system facilitates the development of cellular therapy in China - The Lancet Haematology
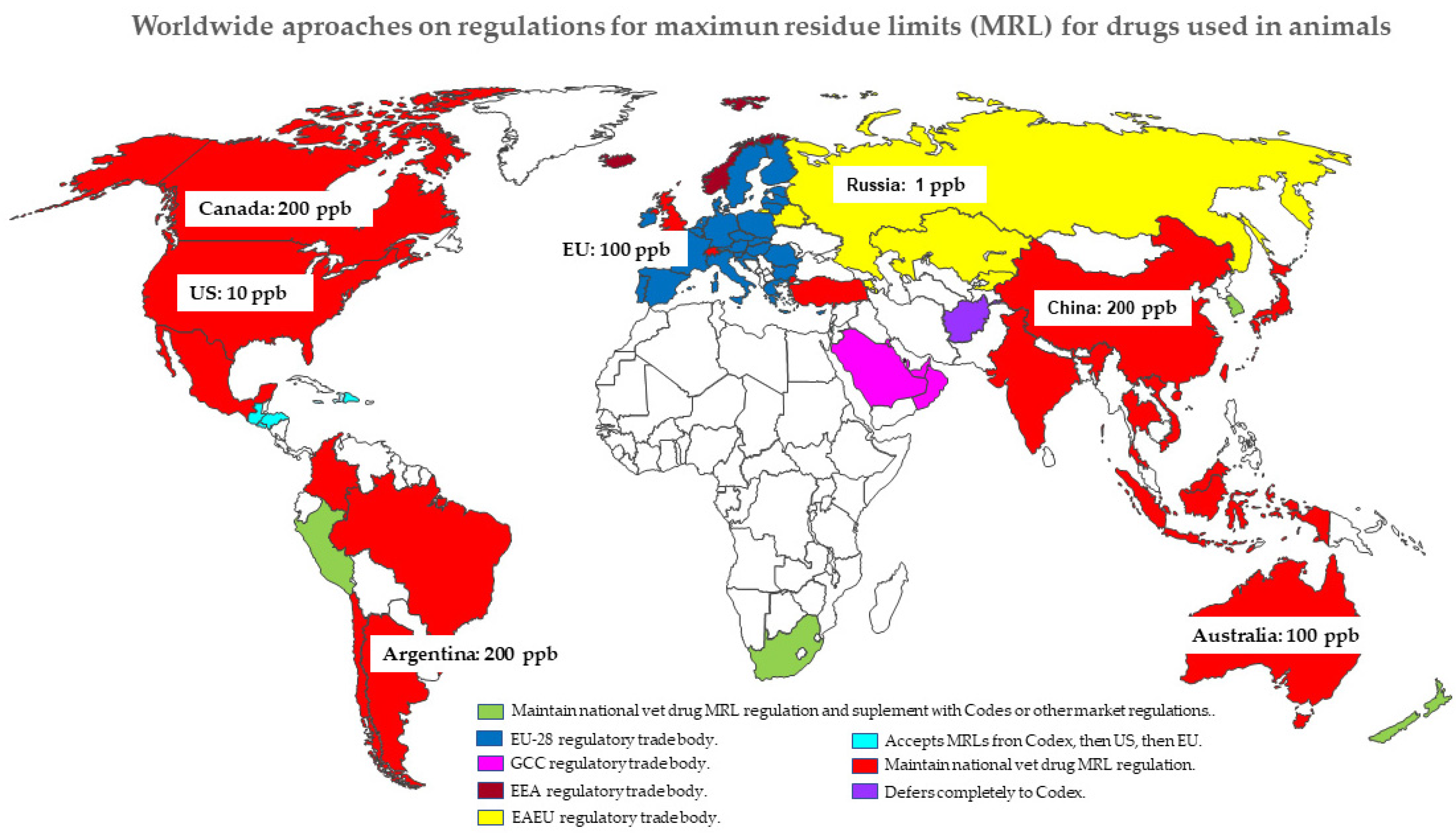
Animals | Free Full-Text | Rational Pharmacotherapy in Infectious Diseases: Issues Related to Drug Residues in Edible Animal Tissues
Commission notice – Application of the Union's pharmaceutical acquis in markets historically dependent on medicines supply f
11107/16 MBT/sy DG C 1 Delegations will find attached document C(2016) 4167 final (Kenya). Encl.: C(2016) 4167 final

Chapter 5 Welcome to the Schengen Hotel in: Immigration and Privacy in the Law of the European Union
EUROPEAN COMMISSION DIRECTORATE-GENERAL FOR HEALTH AND FOOD SAFETY Health systems and products Medical products – quality, saf







